

Vol. 38 (Nº 46) Year 2017. Page 42
Sergey STEPANOV 1; Galina VERETENNİKOVA 2; Olga STEPANOVA 3; Yuliya NİKOLAEVA 4; Olga SVETNEVA 5; Natalia MEYCHİK 6
Received: 27/09/2017 • Approved: 30/09/2017
3. Data, Analysis, and Results
ABSTRACT: The authors have conducted potentiometric titration of carboxylic cation exchanger (degree of crosslinking 12-16%) in a wide range of changes in solution pH (2-12) and concentration NaCl (0.01; 0.1; 0.5; 1 M). The maximum ion exchange capacity of the ion exchanger on Na+ (10.100.088 mmol/g of dry weight) was determined, and it did not depend on ionic strength of the solution. It is shown that in the studied range of NaCl concentrations and pH the process of acid-base balance can be adequately described by the equation of Gregor. The values of the parameters of this equation are calculated, according to which analyzed the behavior of the carboxylic cation exchanger depending on pH and concentration of background electrolyte. The acidic properties of cation exchanger increased with increasing concentration of NaCl from 0.01 to 0.5 M, but further increase in salt concentration did not affect the ionization constant of carboxyl groups. |
RESUMEN: Los autores han llevado a cabo la titulación potenciométrica del intercambiador catiónico carboxílico (grado de reticulación del 12-16%) en una amplia gama de cambios en el pH de la solución (2-12) y concentración de NaCl (0,01; 0,1; 0,5; 1 M). Se determinó la capacidad máxima de intercambio iónico del intercambiador de iones sobre Na + (10.10-0.088 mmol / g de peso seco) y no dependió de la fuerza iónica de la solución. Se demuestra que en el rango estudiado de concentraciones de NaCl y pH el proceso de equilibrio ácido-base puede ser adecuadamente descrito por la ecuación de Gregor. Se calculan los valores de los parámetros de esta ecuación, según los cuales se analizó el comportamiento del intercambiador catiónico carboxílico dependiendo del pH y la concentración del electrólito de fondo. Las propiedades ácidas del intercambiador de cationes aumentaron con una concentración creciente de NaCl de 0,01 a 0,5 M, pero un aumento adicional en la concentración de sal no afectó a la constante de ionización de los grupos carboxilo. |
The study of acid-base properties of ion exchangers with different structure of functional groups is an important step in the study of these polymers (Zharkova, 2016; Tanaka, 2015; Bulat, Volkov & Ilyina, 2016), because, on the one hand, these results serve to confirm the chemical structure of functional groups of the ion exchanger, and on the other, determine the pH range in which these groups are ionized and capable of participating in reactions of ion exchange (Inczédy, 2013; Liang et al., 2013; Nachod & J. Schubert, 2013). Widely used ion exchangers for desalination of water, in analytical chemistry for separation of substances by chromatography method4, chemical engineering (Tanaka, 2015; Rieman & Walton, 2013).
Information about the processes of acid-base balance in the carboxy-containing resins is limited (Zharkova, 2016; Chugunov, 2015). In the famous works of potentiometric curves analyzed in the coordinates pH=f(volume of titrant), and calculate the ionization constants (pKa) – using the equation of Henderson-Hasselbach. On the basis of these studies, the authors of the famous papers (Zharkova, 2016; Chugunov, 2015) consider that the pKa of the functional groups of carboxy-containing ion exchangers in the process of titration changes, and in the ion exchanger there are two types of carboxyl groups, characterized by different values of рКа. However, in the above-mentioned papers did not verify the adequacy of the calculated and experimental curves of potentiometric titration that has been made by the authors the conclusions are clear.
Recently, we developed a new method of synthesis of carboxy-containing high-capacity macroporous cation exchange resin based on acrylate matrix with a high content of functional groups9. In these cation-exchangers when changing the degree of neutralization of the groups and the ionic strength of the surrounding solution is slow to change the conformation of polymer chains and has remained virtually unchanged the degree of swelling during ionization or protonation of carboxyl groups. However, the acid-base properties of these cation exchangers was not determined.
The aim of the present work was the experimental determination of the ionization constants of the carboxyl groups of a new cation exchanger based on a copolymer of acrylonitrile, methyl methacrylate and divinylbenzene by the potentiometric method and assessing the adequacy of calculated and experimental data, determining the dependence of the ionization degree of functional groups from pH and concentration of NaCl.
High-capacity carboxylic cation exchange resin based on acrylate adhesive matrix obtained by radical suspension copolymerization of Acrylonitrile and methyl methacrylate with divinylbenzene (12-16 %) in The limited liability company "Rare earth elements - University of Chemical Technology of Russia according to the method described in (An, Ledovskikh & Balanovskiy, 2008).
Before using cation standardized under dynamic conditions in a cycle of 3% NaOH – H2O – 3% HCI – H2O. The washing with water in the last stage was carried out until the disappearance of CI - in the eluate of the column.
Potentiometric titration was performed by the method of individual batches (Leykin, Meychik & Solovyov, 1978). A dry sample of the normalized cation exchanger at 0.05±0.001 g were placed in glass flasks (volume 50 ml) with glass stopper and poured 20 ml of the NaOH or HCl of various concentrations, but constant ionic strength, which was created by adding the appropriate sodium chloride (background electrolyte). The range of concentrations of acid or alkali in the initial solutions was 0÷10 or 0.1÷0.5 mm, respectively, and the NaCl concentration was 0.01 or 0.1 or 0.5 or 1 M. After 48 hours the samples were separated from the equilibrium solutions in which the pH was determined with an accuracy of ±0.01 (pH Meter, Model 3320, the firm “Jenway” UK), and the concentration of acid or alkali by titration with indicator bromothymol blue. Similarly set the concentration of acid or alkali in the initial solutions. The change in the concentration of Н+ or ОН- in initial and equilibrium solutions was calculated capacity of the cation exchange resin at рНi by the formula:
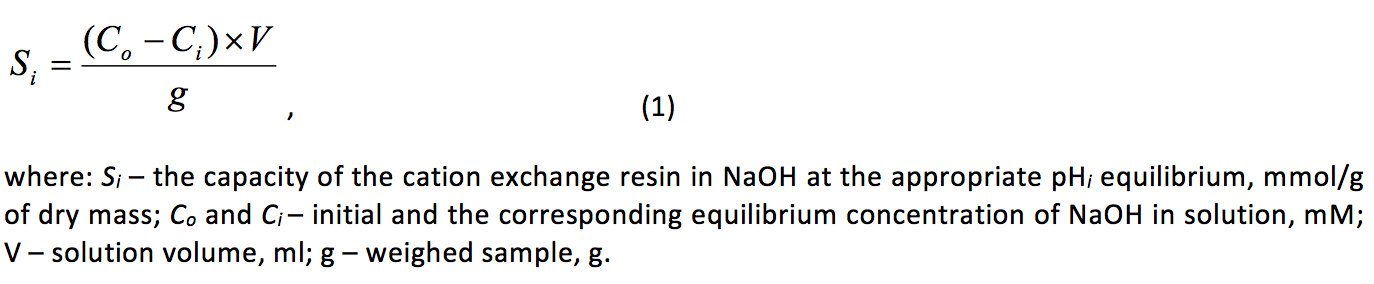
Potentiometric titration at each value of ionic strength of the solution was performed in two repetitions, and for each point of the potentiometric curve – 2-5 analytical replicates. Statistical processing of results was performed using Microsoft Excel and IBM SPSS Statistics.
The curves of potentiometric titration of the studied cation exchange resin are represented by the dependence Si=f(рНi), have monosigmoid character and are characterized by one flexibility point at the coordinates dSi=f(dpHi), that indicates its monofunctionality (Figure 1, an example of a titration at the concentration of NaCl 0.1 М). Regardless of the concentration of the background electrolyte (СNaCl) at рН>10, the capacity of the cation exchanger in relation to the Na+ reached maximum level (Smax), and accounted for 10.10±0.088 mmol/g of dry weight (mean±standard error).
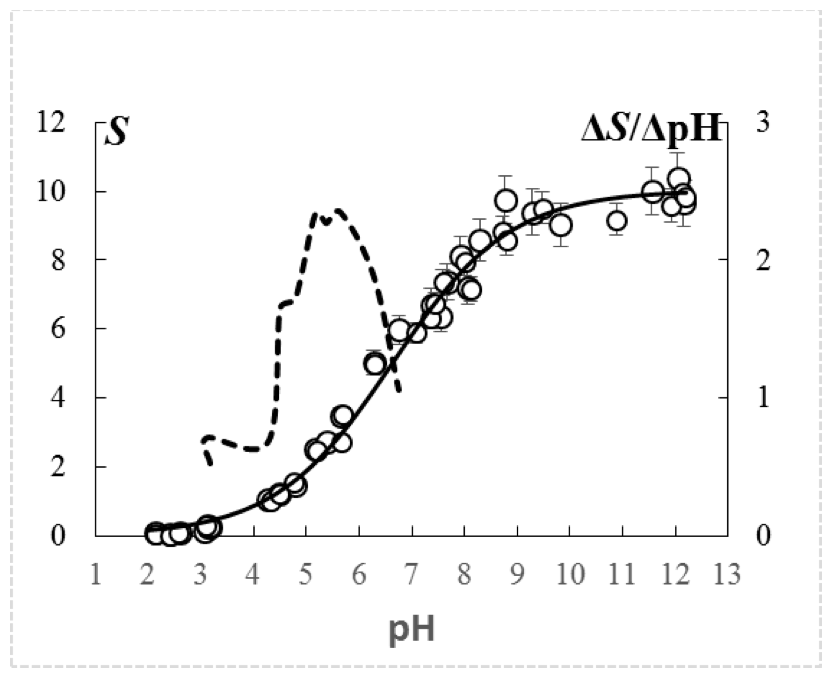
Figure 1. Curve of potentiometric titration of carboxylic cation exchange resin
in the background of 0.1 M NaCI, presented in the form of the dependence S=f(pH).
S – capacity of cation exchange resin for Na+, mmol/g of dry weight of cation exchanger.
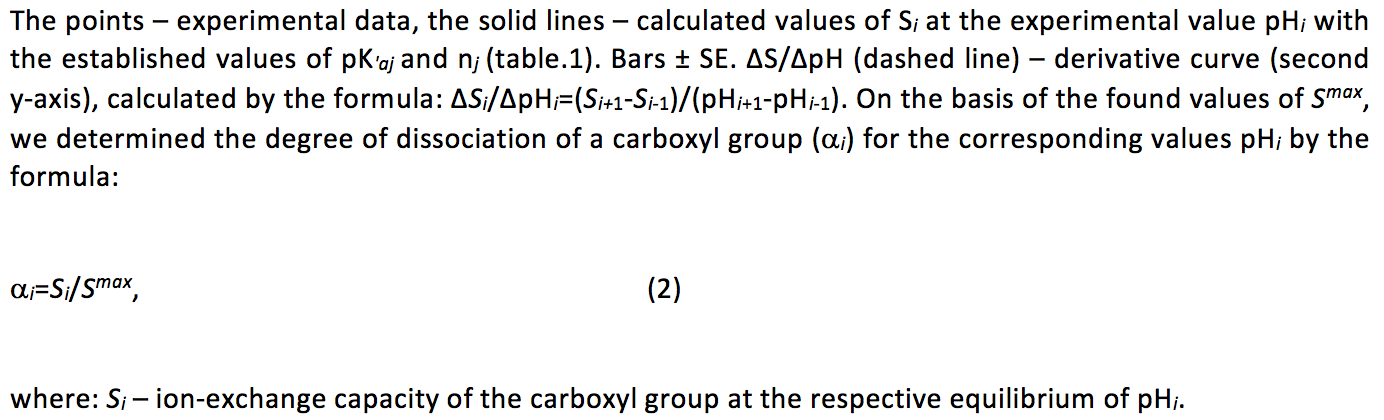

The calculations showed that regardless of the concentration of the background electrolyte the experimental curves with high correlation coefficients linearized in the coordinates of equation 3 (Table. 1), it should be emphasized that the number of points on each straight was 11 or more.
Table 1
The effect of the concentration of the background electrolyte (СNaCI, M)
on the values of the apparent ionization constants (pK'a) and the constant n
with the potentiometric titration of the carboxy-containing cation exchange resin
|
СNaCI |
|||
0.01 |
0.1 |
0.5 |
1 |
|
pK'a |
8,08±0,02 |
6.62±0,02 |
5.72±0,02 |
5.85±0,04 |
n |
1.48±0,15 |
2.54±0,03 |
2.60±0,09 |
3.12±0,07 |
r |
0.965 |
0.991 |
0.997 |
0.979 |
Footnote. Calculations of pK'a and n were conducted in accordance with
the equation pHi=pK'a+nlg(ai/(1–ai)), ai is the degree of dissociation of
the carboxyl group with appropriate value of рНi. r – coefficient of dependency
correlation pHi =f(lg(ai/(1–ai)). We gave the average values ± standard deviation.
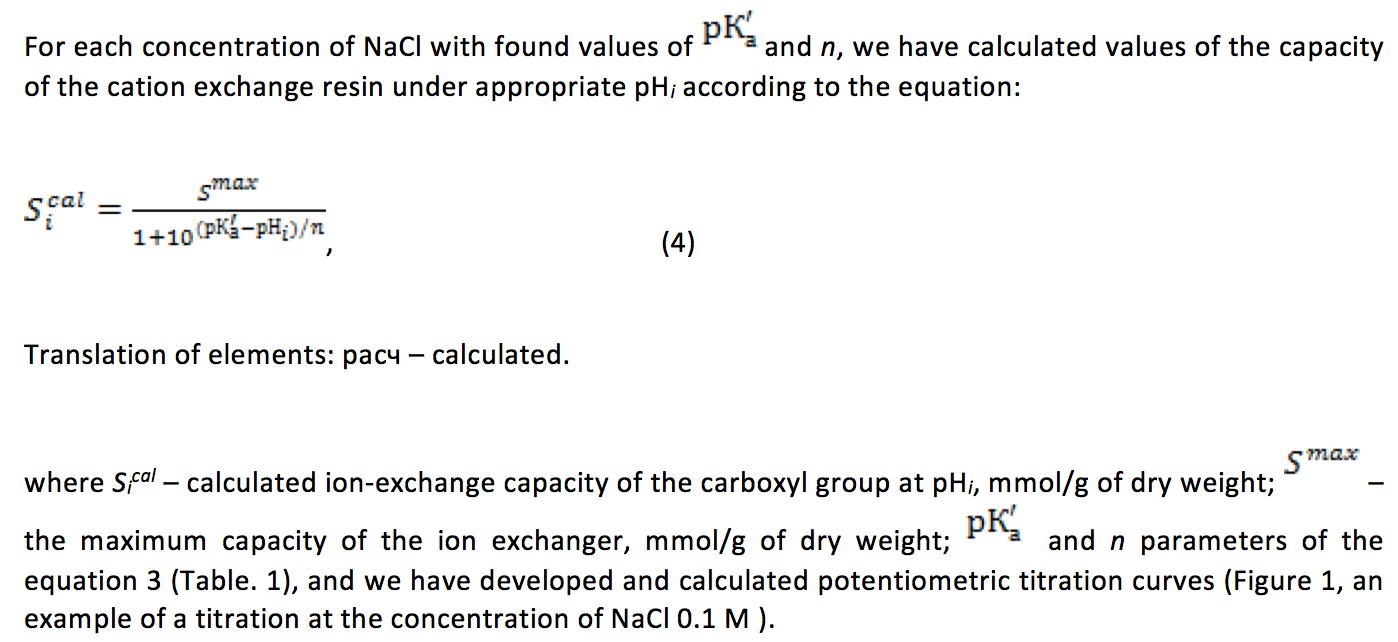
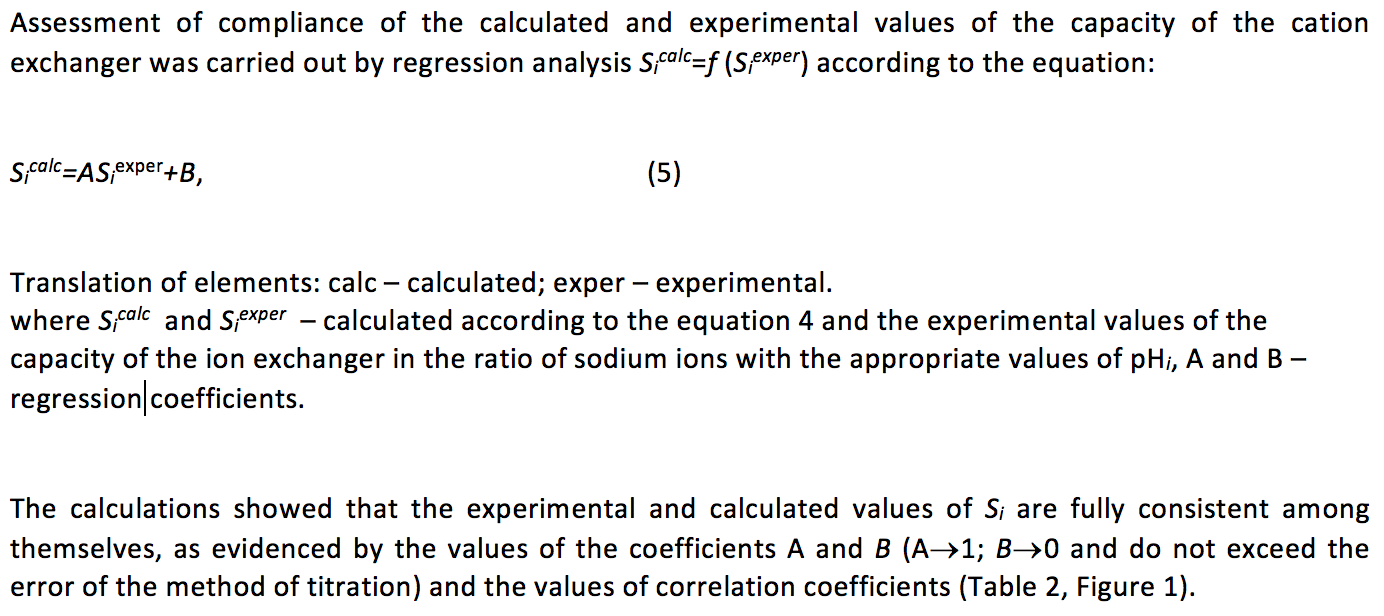
Table 2
The adequacy of the experimental and calculated potentiometric titration of the
carboxyl-containing cation exchange resin with different concentrations of NaCI in
the solution (СNaCI, M). A and В – regression parameters of the equation
Sicalc=АSiexper+В, where Sicalc and Siexper – calculated according to the
equation 4 and the experimental values of the capacity of the ion exchanger
at the appropriate values of рНi. r – correlation coefficient of relation of Sicalc=f(Siexper)
|
СNaCI |
|||
0.01 |
0.1 |
0.5 |
1 |
|
А |
0.983 |
1.018 |
1.018 |
0.991 |
В |
0.216 |
0.005 |
0.114 |
0.112 |
r |
0.987 |
0.986 |
0.994 |
0.981 |
In accordance with the results, the increase in the concentration of background electrolyte from 0.01 to 0.5 M leads to a decrease in pK'a values of carboxyl groups in the ion exchanger, i.e. increase their acidic properties, which is characteristic of carboxylic cation exchangers. However, a further increase of СNaCI up to 1 M does not affect this parameter (Table. 1). For carboxylic cation-exchangers with a low degree of cross-linking (less than 2%) it is known that the value of pK'a depends on the concentration of background electrolyte, and at 1 M concentration of the latter is approximately equal to pK'a of linear polyacid (Gelferich, 1962; Meychik & Yermakov, 2001) but for carboxylic ion exchangers with a high degree of crosslinking (more than 5%) such dependence was found for the first time. The difference is that in the studied carboxylic cation, the change in the values of pK'a with the concentration background less in compared with weakly crosslinking ion exchangers, and the resulting value of pK'a 5.85 in 1M of NaCI (Table. 1) is higher than the value of this indicator for polyacrylic acid (Inczédy, 2013; Leykin, Meychik & Solovyov, 1978; Shataeva, Kuznetsova & Elkin, 2001).
In works (Zharkova, 2016; Chugunov, 2015), the study of acid-base equilibrium of the carboxyl ion exchangers was carried out with different salts (NaNO3, NaCl), but at their fixed concentration in solution. Obtained in the present work, the values of pK'a for the carboxylic cation exchanger (6.62 in 0.1 M of NaCI and 5.85 in 1 M of NaCI, Table 1) at respective concentrations of background electrolyte are close to those described in the literature. Therefore, in 0.1 M of NaNO3 for cation Tokem-250, the value of pK'a totaled 6.591 and KB-2E – 5.92 in 1M of NaCl8.
Noteworthy is a significant effect of concentration of salt in the solution that changes the constant n of equation 3 (Table. 1). Changes in the value of this index appear at lowering the amount of СNaCI from 0.1 to 0.01 and increasing from 0.5 to 1 M, while at the concentration range of 0.1-0.5 M, the values of n were not significantly different (p<0.05) and equal to 3.12. In accordance with existing conceptions, n is a constant depending on the structure of the polymer matrix and the nature of counter ion (Shataeva et al., 1979), but in our experiment, these two variables remained unchanged. It can be assumed that changes in the parameter n were associated with different trends in the change in free energy of the macromolecule in its neutralization in solutions with СNaCI<0,1 and СNaCI>0,5 М. In one case, it decreases and in the other – increases.
Based on the experimental data, it is possible to build the estimated dependency curves S =f (pH) (Figure 2), which clearly show that the investigated carboxylic cation exchange resins are monofunctional and do not have second ionogenic groups with pK'a ~ 1.5, which is expected based on the study of acid-base equilibrium of the carboxylic cation exchange resin (brand Tokem-200) at the ionic strength of the solution 1.0 M8. The results of this study show (Figure 2) that in the acidic area (pH 1-2), the carboxyl groups are not fully deprotonated, because at these pH, the capacity of the cation exchanger according to Na+ ranges from 0.2 to 0.4 mmol/g of dry weight that is not due to dibasic carboxyl groups, but due to the change in electrostatic free energy of a macromolecule in a 1 M solution of NaCl. Evidence of this situation is the results of potentiometric titration of the studied carboxylic cation exchange resin in solutions of NaCl c concentrations of 0.01 and 0.1 M. Under these conditions, the carboxylic groups are fully protonated, because the capacity of the cation exchanger according to Na+ tends to 0 (Figure 2).
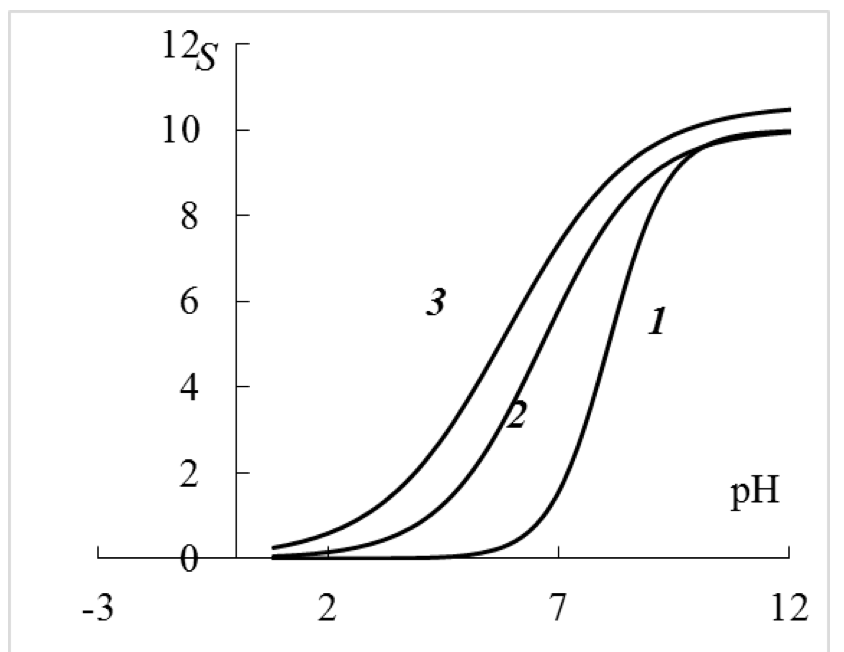
Figure 2. The calculated titration curves (equation 4 according to Table.
1) of carboxylic cation exchange resin in the background of various NaCI concentrations:
1 – 0.01 M; 2 – 0.1 M; 3 – 1 M. S – capacity of cation exchange resin for Na+,
mmol/g of dry weight of cation exchange resin.
Thus, in the present work new data on the regularities of the processes of acid-base equilibrium occurring in the cross-linked macroporous carboxylic cation exchange resin based on acrylate matrix. For these polymers, as well as for the linear analogues, the pK'a of carboxyl groups depends on the concentration of salt in the environment but its value is higher than for carboxyl linear polyacids. The parameter n in the Henderson-Hasselbach equation modified by Gregor is determined not only by the degree of crosslinking of the ion exchanger and the nature of the ion, which is filled with sorbent, but also by the concentration of the electrolyte in which the potentiometric titration of carboxylic cation exchangers is carried out.
The work is done in the framework of the Agreement on granting subsidies of 19.08.2015 No. 14.579.21.0099 (identification number of the project RFMEF15791X0099) funded by the Ministry of education and science of the Russian Federation.
Zharkova, V.V. (2016). Dissertation by Candidate of Chemical sciences, Tomsk: Federal state Autonomous educational institution of higher education.
Tanaka, Y. (2015). “Ion exchange membranes: fundamentals and applications”, Elsevier.
Bulat, P.V., Volkov, K.N., & Ilyina, T.Y. (2016). IEJME-Mathematics Education, 11(8), 2949-2962.
Inczédy, J. (2013). “Analytical applications of ion exchangers”. Elsevier.
Liang, P., Yuan, L., Yang, X., Zhou Sh., & Huang, X. (2013). Water research, 47(7), 2523-2530.
Nachod, F.C., & Schubert, J. (2013). “Ion exchange technology”, Academic Press.
Rieman, W., & Walton, H.F. (2013). “Ion Exchange in Analytical Chemistry: International Series of Monographs in Analytical Chemistry”, Elsevier.
Chugunov, A.S. (2015). Chemistry and technology of inorganic substances, In Proceedings of Saint Petersburg State Technological Institute (TU), No. 29, pp. 19.
An, E.D., Ledovskikh, G.I., Balanovskiy, N.V. (2008). RU Patent 2326130.
Leykin, Yu.A., Meychik, N.R., & Solovyov, V.K. (1978). Russian J. Phys. Chem., 52, 1420.
Shataeva, L.A., Kuznetsova, N.N. & Elkin, G.E. (1979). “The carboxyl ion in biology”, Leningrad: Nauka.
Gelferich, F. (1962). “Ion exchange resins”, Moscow: Foreign literature.
Meychik, N.R., & Yermakov, I.P. (2001). Plant and Soil, 234,181.
1. D.Mendeleev University of Chemical Technology of Russia, Moscow, RUSSIA. E-mail: gveretennkova@yahoo.com
2. Lomonosov Moscow State University, Moscow, RUSSIA
3. Rare earth elements – UCTR” LLC, Moscow, RUSSIA